Real results in flu protection you can count on.
Flublok® and Fluzone® High-Dose not only help protect you from flu illness, but they are also backed by some of the largest and most robust flu vaccine studies so you can have confidence in their efficacy and safety.
With 4x the dose, Fluzone High-Dose provided 24% better protection compared to a regular flu shot*
The ability of Fluzone High-Dose to protect those 65+ from flu-related hospitalizations, including hospitalizations due to cardiovascular events and respiratory events, was assessed over 12 flu seasons in comparison to regular flu shots
Flublok demonstrated 30% better flu protection when compared to Fluarix® Quadrivalent in adults 50+†
In [ the largest ] randomized real-world flu effectiveness study to date (~1.6 million poeple), Flublok provided better flu protection in adults 50-64 compared to a regular flu shot
Flublok is supported by one of the largest safety studies of a flu vaccine in pregnant women, including >15,000 patients
See full study design and limitation details below.
For Fluzone High-Dose, the most common side effects were slightly more frequent compared to the regular flu shot and included pain where you got the shot, muscle ache, tiredness, and headache.
The safety profile of Flublok was similar to that of the regular flu shot in adults aged 50+. In adults 50+, the most common side effects were tenderness and/or pain where you got the shot, headache, and tiredness.
*Based on a clinical trial of ~32,000 adults 65+ conducted during 2 flu seasons, 2011-2012 and 2012-2013, where an influenza case was laboratory tested.
†Randomized, controlled clinical trial of ~9,000 adults 50+ conducted during 2014-2015 flu (or influenza) season.
‡Analysis of data captured during day-to-day medical practice of Medicare fee-for-service claims collected from 12.7 million adults aged 65+ during the 2019-2020 influenza season. Specific characteristics of 2019-2020 season, such as varied strain circulation, may have impacted results.
FLUZONE® HIGH-DOSE (INFLUENZA VACCINE)
FLUZONE® High-Dose (Influenza Vaccine) is a flu vaccine approved by the Food and Drug Administration (FDA) and has been proven to provide superior flu protection compared to a standard-dose flu shot in adults 65 and older.
Studied for more than a decade, Fluzone® High-Dose
has shown its ability to provide better protection against serious
Higher dose provides higher protection: With 4x the dose, Fluzone® High-Dose provides 24% better protection compared to a regular flu shot.

Study Design: Based on a clinical trial of ~32,000 adults 65+ comparing Fluzone High-Dose with Fluzone conducted during 2 flu seasons, 2011-2012 and 2012-2013, where an influenza case was laboratory tested.
The primary study results met a strict definition of “superior” set by the health authority before the study was conducted.
Compared with FLUZONE®, in adults 65 years of age and older, the most common side effects were pain where you got the shot, muscle pain, tiredness, and headache. Other side effects may occur.
In an analysis of published studies, people vaccinated with Fluzone® High-Dose experienced fewer flu-related hospitalizations compared with those vaccinated with standard-dose flu shots.
14.3 %
fewer influenza-like illnesses§
12.8 %
fewer hospitalizations due to cardiovascular events
14.7 %
fewer hospitalizations due to respiratory events


8.2 %
fewer hospitalizations
11.2 %
fewer hospitalizations due to flu


16.7 %
fewer hospitalizations due to cardiorespiratory events
10.4 %
fewer hospitalizations/ER visits due to flu
Note: Select endpoints are presented here; relative vaccine effectiveness against pneumonia was also studied.
Study Design: Association of these clinical events with Fluzone® High-Dose was based on a systematic review of studies in more than 45 million adults aged 65+, conducted during 12 influenza seasons (2009-2010 to 2019-2020, and 2021-2022).
Study was funded and performed by Sanofi.
Study Limitations: Percentages are approximate and results may have been influenced by other variables. Results are pooled analysis from multiple studies; individual study results may vary.
§
Defined as visits with a rapid influenza diagnostic test followed by prescription of antiviral medication.
For Fluzone High-Dose, in adults 65 years of age and older, the most common side effects were pain where you got the shot, muscle pain, tiredness, and headache.
FLUBLOK® (INFLUENZA VACCINE)
In a clinical trial in adults 50 years and older, vaccination with Flublok® (Quadrivalent) was compared to Fluarix® Quadrivalent.
The effectiveness of Flublok® was also studied using data from general day-to-day medical practice among more than 12 million adults 65 years and older. Vaccination with Flublok was also compared to standard-dose flu vaccines. The results were:
Better flu protection
when compared to Fluarix®
Quadrivalent in adults 50+.
Study Design: Randomized, controlled clinical trial of ~9,000 adults 50+ conducted during 2014-2015 flu (or influenza) season.
In adults 50+, the most common side effects were pain and/or tenderness at the injection site, headache, and tiredness.
The efficacy of the trivalent and quadrivalent formulations are related because both are made using the same process and have overlapping compositions.
In a real-world evidence study, people who received Flublok experienced 31% fewer flu-related hospitalizations compared to regular flu shots.
Study Design: Real-world evidence study in approximately 15,000 patients aged 18+ years to assess the impact of Flublok Quadrivalent on flu-related hospitalization vs a range of cell- and egg-based regular flu shots. The efficacies of the trivalent and quadrivalent formulations are related because both are made using the same process and have overlapping compositions.
Study Limitations: The study population was representative of the adult population of Allegheny County, where the study was conducted, with 79% being white and 51% being female.
The hospitals used a well-connected system that regularly updated vaccination information from the state registry, ensuring accurate records of who was vaccinated.
If a patient’s flu shot was not recorded in the hospital system or state registry, they might have been incorrectly labeled as unvaccinated
The study only looked at hospitalizations, so it might have missed milder flu cases that did not require hospital care.
Doctors might have been more likely to test unvaccinated people for flu, which could make it seem like more unvaccinated people got sick.
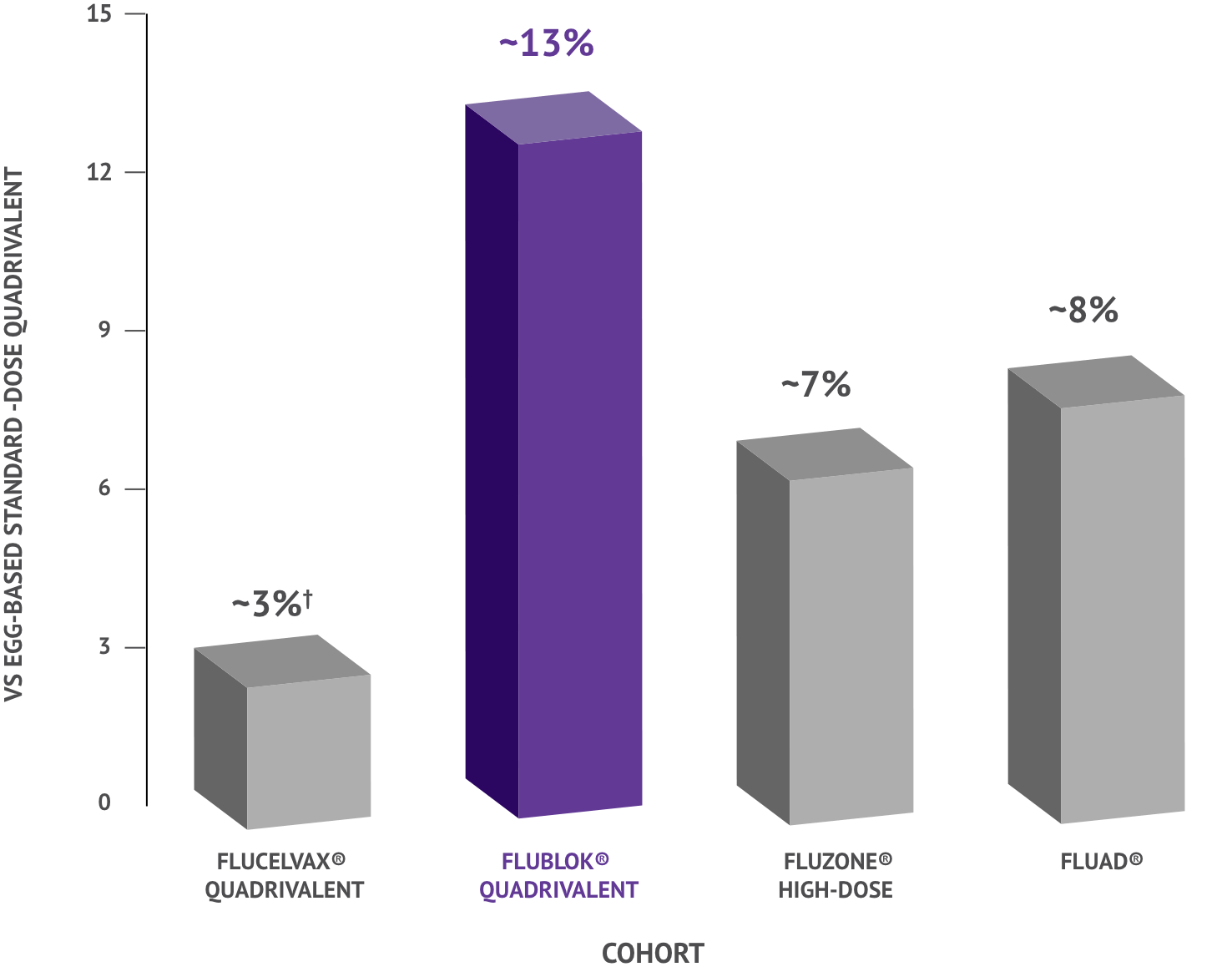
In a review of flu vaccines, FLUBLOK (Quadrivalent) demonstrated 13% fewer flu-related hospitalizations and emergency room visits compared to standard-dose flu shot in adults 65+.

||
Flucelvax® Quadrivalent was not statistically significant.
The data shown is 1 of 3 primary analyses. Two additional primary analyses were conducted: 2 vaccine analyses comparing Flucelvax® Quadrivalent with standard-dose quadrivalent and Flublok® (Quadrivalent) with standard-dose quadrivalent.
Study Design: Analysis of data captured during day-to-day medical practice of Medicare fee-for-service claims collected from 12.7 million adults aged 65+ during the 2019-2020 influenza season. Specific characteristics of 2019-2020 season, such as varied strain circulation, may have impacted results.
Study conducted by the FDA and Centers for Medicare and Medicaid Services.
Study Limitations: Lack of information about the specific virus that caused the illnesses may have resulted in underestimating differences between the vaccines.
Biases may have affected the results.
The study period was cut off at the end of February 2020 to avoid potential bias from the overlap between flu season and the escalation of the COVID-19 pandemic in the US.
Flublok showed no increased risk of major birth defects and miscarriages in pregnant people.
Study Design: Analysis of data on ~15,000 pregnant individuals, including those with chronic conditions, who were exposed to Flublok during 28 days prior to conception or throughout their pregnancy. Data showed no increased risk of miscarriages or major birth defects with vaccine exposure.
The study was conducted during the 2018-2019 and 2019-2020 flu seasons and assessed specific pregnancy outcomes; not all possible impacts were assessed.


